What is the Oncotype DX® Breast Cancer Test?
LANDMARK TAILORx RESULTS
The long-awaited results of the Trial Assigning IndividuaLized Options for Treatment (TAILORx) definitively identified a vast majority of women with early stage breast cancer who will receive no benefit from chemotherapy, and the minority for whom chemotherapy can be life-saving.
Overview
Oncotype DX Breast Recurrence ScoreThe Oncotype DX assay is a 21-gene assay that predicts the likelihood of chemotherapy benefit and 10-year risk of distant recurrence to inform adjuvant treatment decisions in certain women with early-stage invasive breast cancer.
The Oncotype DX Assay:
- Supports your treatment decisions - The Oncotype DX assay is incorporated in the ASCO®, NCCN®, St. Gallen and ESMO® guidelines, and is recommended by NICE for use in clinical practice to guide adjuvant chemotherapy treatment decisions for certain patients with early-stage breast cancer, including micrometastatic disease. Access this guidance.
- Informs treatment decisions and improves confidence in treatment decisions for physicians and patients. Studies suggest that the results of the assay change breast cancer treatment decisions in approximately 30% of cases. 1–8
- Offers expanded clinical utility-Enhanced reports now include quantitative ER, PR and HER2 values.
Extensively Validated and Ordered
- The clinical value of the Oncotype DX assay was shown in clinical studies involving nearly 4,000 patients
- Over 900,000 patients worldwide have received the information provided by the Oncotype DX assay
- Over 19,000 physicians have ordered the Oncotype DX assay in over 90 countries
- Genomic Health is a CLIA-certified, CAP-accredited reference laboratory and is a leader in the field of cancer genomics
- Clinical validation
Clinical Utility
The Oncotype DX assay provides clinical experience information:
- For pre- and post-menopausal women with node-negative, hormone-receptor-positive invasive breast cancer
- For post-menopausal women with node-positive, hormone-receptor-positive invasive breast cancer
AND
Which patients may benefit from the Oncotype DX Breast Recurrence Score® test?
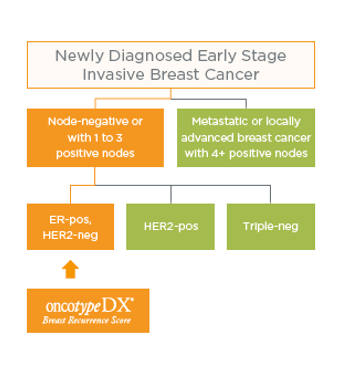
NICE Diagnostic Guidance* on the use of the Oncotype DX Breast Recurrence Score test for NHS patients

Update NICE Breast Cancer Quality Standard [QS12] recommends Oncotype DX testing. (June 2016, www.nice.org.uk
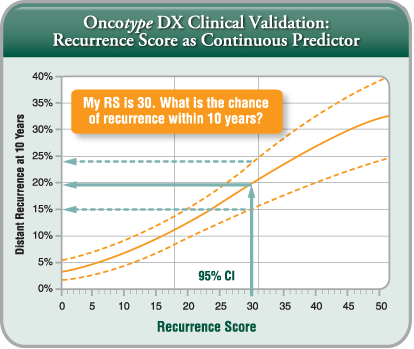
What is the Recurrence Score® result?
Because breast cancer is a heterogeneous disease, it is important to have an assay that provides a continuous view of the biology that can capture the risk of individual patients. The Recurrence Score is a continuous score that provides an individual estimate of the 10 year risk of distant recurrence and predicts the likelihood of benefit from chemotherapy. The quantitative nature of PCR allows for a continuous score as opposed to a binary result (low vs. high only). This method is designed to provide you and your patients with an individual score and to help inform your treatment plan.
Underlying Technology
The Oncotype DX Assay Process
As a multi-gene diagnostic assay, the Oncotype DX assay is designed to support individualised treatment planning. The assay provides a quantitative assessment of the likelihood of chemotherapy benefit and distant recurrence, which may increase confidence that the treatment plan is tailored to the individual patient.
The Oncotype DX assay is performed in the licensed Genomic Health® laboratory where the assay was developed. First, RNA is extracted from the breast cancer tumour specimen and purified. Next, the RNA is analysed using a technique called real-time RT-PCR (reverse transcriptase-polymerase chain reaction). Finally, the Recurrence Score® result is calculated from the gene expression results.
Advantages of RT-PCR
The Oncotype DX assay analyses the expression of a panel of 21 genes from a tumour specimen using a technique called RT-PCR. A high-throughput, real-time RT-PCR method was developed to analyse the expression of select genes simultaneously. It is sensitive, specific, highly reproducible and has a wide dynamic range. RT-PCR is a mature technology that is routinely utilised in several clinical applications including viral load testing for HIV.
To quantify gene expression, RNA is extracted from formalin-fixed, paraffin-embedded (FPET) tumour tissue and subjected to DNase I treatment. Total RNA content is measured and the absence of DNA contamination is verified. Reverse transcription is performed and is followed by quantitative TaqMan® (Roche Molecular Systems, Inc.) RT-PCR reactions in 384-well plates. The expression of each of 16 genes is measured in triplicate and then normalised relative to a set of five reference genes.
The Oncotype DX assay standardised testing methods have been optimised to minimise variability due to:
- Tissue preparation method: FPET vs. fresh frozen
- Tumour block age, storage and variability in preparation
- Heterogeneity within and between FPET blocks
- Heterogeneity with respect to enriched tumour and non-tumour areas within an FPET block
Oncotype DX Assay Development
The Oncotype DX assay was developed in four steps. Each of these steps is described in detail below.
- Optimisation of methods for quantifying gene expression in formalin-fixed, paraffin-embedded tissue (FPET)
- Selection of 250 candidate genes from the human genome
- Testing of candidate genes to identify an optimal gene panel for clinical validation
- Prospective clinical validation of the 21-gene panel and Recurrence Score result calculation
Step 1. Optimisation of methods for quantifying gene expression in formalin-fixed, paraffin-embedded tissue
The ability to work with FPET samples is critical, as this is the standard method for tumour preservation and storage in many countries. When tissue is preserved in paraffin, the RNA is fragmented. However, the relative ratio of RNA between genes is unchanged. By utilising RT-PCR techniques, the expression of most genes—relative to a set of reference genes–can be measured. To develop the Oncotype DX assay, Genomic Health researchers optimised RT-PCR technology 1) for high-throughput, real-time quantitation of specific RNA in FPET, and 2) to be reproducible regardless of the variability inherent in tumour blocks.
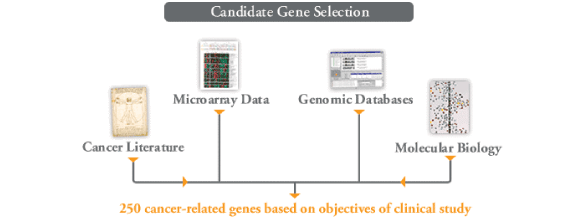
Step 2. Selection of 250 candidate genes from the human genome
Genomic Health researchers relied on numerous sources to identify 250 candidate genes—those possibly associated with breast cancer tumour behavior—from among the approximately 25,000 genes in the human genome.
Step 3. Testing of candidate genes to identify an optimal gene panel for clinical validation
The 250 candidate genes were analysed in a total of 447 patients from three independent clinical studies in order to identify a panel of genes strongly correlated with distant recurrence-free survival. The selection of the 16 cancer genes used for the Oncotype DX assay was based on the results of the three clinical trials, which demonstrated a consistent and strong statistical link between these genes and distant breast cancer recurrence. Five reference genes were identified to normalise the expression of these cancer-related genes. In addition, these studies were the basis for the Recurrence Score result calculation, which combines the gene expression data from this gene panel into a single result.
Step 4. Prospective clinical validation of the 21-gene panel and Recurrence Score result calculation
The Oncotype DX assay gene panel and Recurrence Score result calculation were validated in a large, independent, multicenter clinical trial (NSABP Study B-14) and in a large population-based case-control study in breast cancer patients at Northern California Kaiser Permanente. The endpoints and analysis plan were prospectively defined. The results of these studies were included in the Best of Oncology session at ASCO 2004 and the results of the NSABP B-14 clinical validation study were published in The New England Journal of Medicine (December 30, 2004).
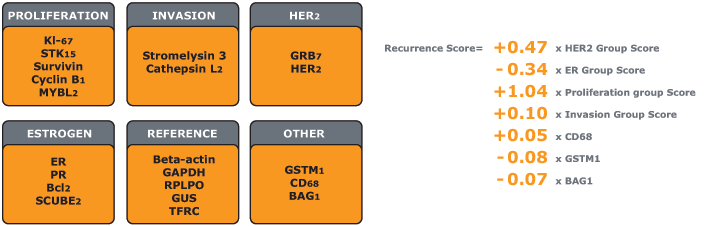
21-Gene Panel Used to Calculate Recurrence Score Result
The Oncotype DX assay gene panel was selected from experiments based on DNA arrays performed on fresh-frozen tissue. The Recurrence Score result algorithm was derived from 3 studies of breast cancer patients and then was clinically validated in multiple independent studies.
- The Recurrence Score result is calculated from the expression of 16 cancer-related genes and 5 reference genes used to normalise the expression of those cancer genes
- In clinical studies, the 16 cancer genes demonstrated a consistent statistical link to distant breast cancer recurrence, as well as robust predictive power regarding chemotherapy benefit
21-Gene Panel Used to Calculate Recurrence Score Result:
16 cancer genes and
5 reference genes from
3 studies
Quantitative Single-Gene Scores
The Oncotype DX assay report now includes quantitative scores for oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) gene expression by RT-PCR (all reports generated as of September 20, 2008). ER, PR and HER2 are three of the genes measured in the determination of the Recurrence Score result.
ER and PR
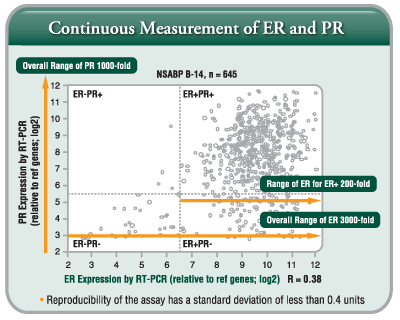
- Continuous Measurement by RT-PCR:
ER/PR status is critical in determining the use and estimating the benefit of adjuvant hormonal therapy. Breast cancers show a broad range of hormone receptor expression, and RT-PCR is able to accurately convey this continuum. RT-PCR is able to measure ER and PR as a continuous distribution of gene expression over a 3,000-fold and 1,000-fold range, for ER and PR, respectively.
A quantitative ER Score can provide further insight into treatment decisions by helping to determine the magnitude of tamoxifen benefit for an individual patient (the higher the ER score, the greater the likelihood of tamoxifen benefit).
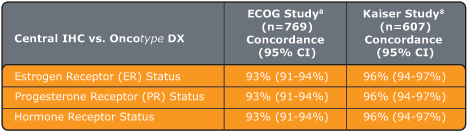
- Concordance:
The Oncotype DX assay determines ER and PR status (positive or negative) by measuring gene expression at the RNA level. There is a high concordance between ER and PR status as determined by the Oncotype DX assay and by immunohistochemistry (IHC), which measures ER and PR gene expression at the protein level.
Studies were performed with ECOG (E2197 study, 769 patient samples) and Northern California Kaiser Permanente (607 patient samples):
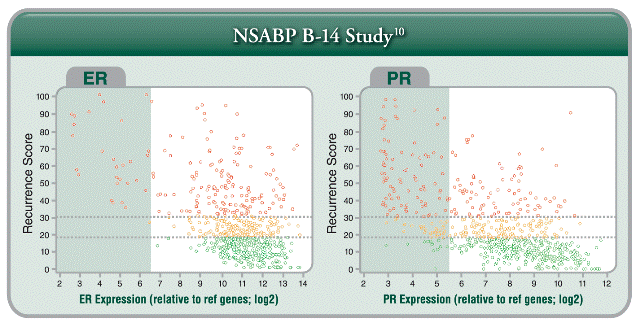
- The Relationship Between ER/PR and the Recurrence Score Result: The Recurrence Score result can be either low or high for high ER levels. The Recurrence Score result combines the ER level (related to response to tamoxifen) with the expression of other genes. The Recurrence Score result is a better predictor of distant recurrence at 10 years given 5 years of tamoxifen therapy in the ER+ population than ER/PR status is alone.
HER2
HER2 is an important marker for therapeutic decision-making for patients with breast cancer, and its measurement significantly impacts the chosen course of treatment.
- Quantitative HER2 Information:
A HER2 Score by the Oncotype DX assay is another measure for clinicians and patients to determine HER2 status, and it provides further insight into individual patients’ breast cancer tumour biology, although the Recurrence Score result remains the best tool for assessing prognosis and prediction of chemotherapy benefit. - Highly Concordant with IHC and FISH: The Oncotype DX assay’s concordance with IHC and FISH meets or exceeds the ASCO®/CAP guidelines requirement of 95% for determination of HER2 status.
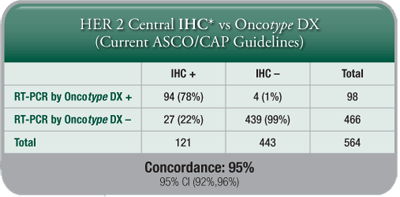
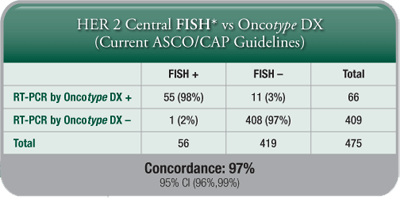
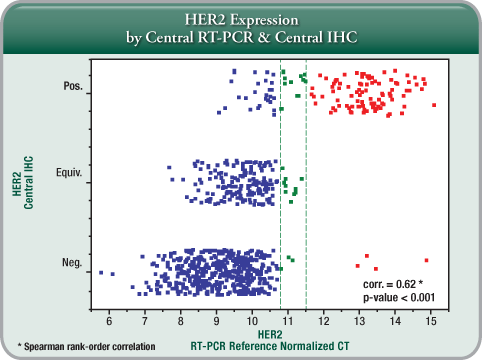
- Added Clinical Information:
Many clinicians who rely on the Oncotype DX assay for treatment planning have requested that Genomic Health also report a quantitative HER2 Score as this is already a component of the Recurrence Score result.
– To provide further clarification especially in cases of equivocal IHC and/or FISH results or discordance between FISH and IHC. - Addressing a Limitation with Current Testing:
The impact of preanalytical variability can be minimised by "normalisation" strategies used in quantitative gene expression assessment as performed by quantitative RT-PCR by the Oncotype DX assay.
Expanded Use of the Oncotype DX Assay for Node-Positive Patients
The Oncotype DX assay provides recurrence risk information for both node-negative and node-positive patients9
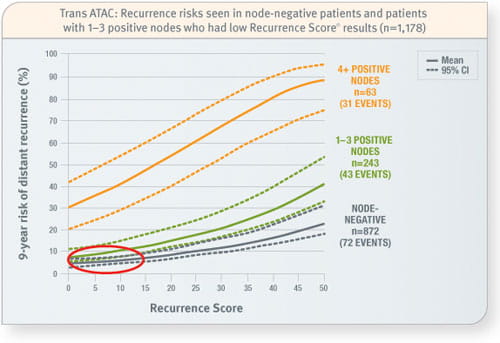
Trans ATAC
Prospective analysis of archived tissue from 1,231 postmenopausal patients with invasive breast cancer treated with tamoxifen or an aromatase inhibitor, of whom 1,178 were ER-positive and either node-negative or node-positive. Of 306 node-positive patients: 79% had 1–3 positive nodes, 21% had 4 or more positive nodes, and 4% had unknown nodal status. The mean age was 64 years and 67% of tumours were ≤ 2.0 cm in size.
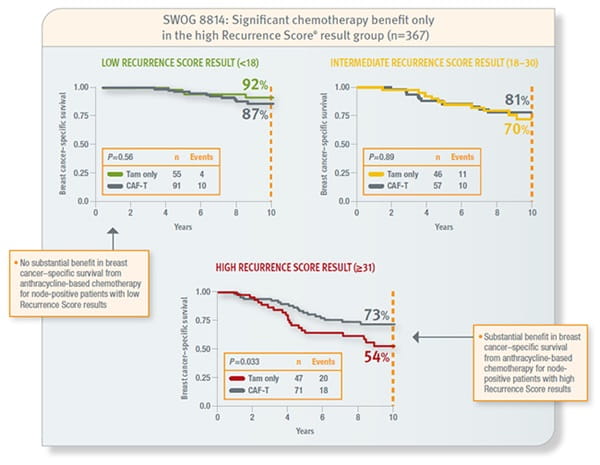
SWOG 8814
Prospective analysis of archived tissue from 367 postmenopausal, hormone receptor–positive, node-positive patients with invasive breast cancer treated with tamoxifen or tamoxifen plus CAF. Approximately 62% had 1–3 positive nodes and the remainder had 4 or more. Mean age was 60 years (range 42–81), 20% were PR–negative, and 64% of tumours were 2–5 cm in size.
References:
1. Asad J, Jacobson A, Estabrook A et al. Does Oncotype DX recurrence score affect management of patients with early-stage breast cancer? Am J Surg. 2008; 196: 527–529.
2. R Oratz, D Paul, AL Cohn, SM Sedlacek et al. Impact of a Commercial Reference Laboratory Test Recurrence Score on Decision Making in Early-Stage Breast Cancer. J Oncol Pract. 2007; 3(4):182-186.
3. Partin JF, Mamounas EP. et al. Impact of the 21-Gene Recurrence Score Assay Compared With Standard Clinicopathologic Guidelines in Adjuvant Therapy Selection for Node-Negative, Estrogen Receptor-Positive Breast Cancer. Ann Surg Oncol. 2011 May 3. [Epub ahead of print]
4. J. Albanell, A. Gonzalez, M. Ruiz-Borrego, et al. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER1) node-negative breast cancer Ann Oncol. 2011 Jun 6. [Epub ahead of print]
5. SS Lo, PB Mumby, J Norton, K Rychlik, J Smerage, J Kash, HK Chew, ER Gaynor, DF Hayes, A Epstein, KS Albain. Prospective Multicenter Study of the Impact of the 21-Gene Recurrence Score Assay on Medical Oncologist and Patient Adjuvant Breast Cancer Treatment Selection. J Clin Oncol. 2010; 28(10):1671-1676.
6. Ruth Oratz, MD, FACP, Benjamin Kim, MD, MPhil, Calvin Chao, MD, Stanley Skrzypczak, MS, Caron Ory, RN, MSN, Roberto Bugarini, DStat, and Michael Broder, MD, FACOG Physician Survey of the Effect of the 21-Gene Recurrence Score Assay Results on Treatment Recommendations for Patients With Lymph Node–Positive, Estrogen Receptor–Positive Breast Cancer J Oncol Pract. 2011; Journal of Oncology Practice 2011.
7. Geffen DB, Abu-Ghanem S, Sion-Vardy N, Braunstein R, Tokar M, Ariad S, Delgado B, Bayme M, Koretz M. The impact of the 21-gene recurrence score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy. Ann Oncol. 2011 Mar 1. [Epub ahead of print]
8. Henry L, Stojadinovic A, Swain SM et al. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol. 2009; 99: 319–323.
9. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene Recurrence Score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC Study. J Clin Oncol. 2010;28:1829-1834.
10. Albain K, Barlow W, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55-65.
American Society of Clinical Oncology (ASCO) and ASCO are registered trademarks of ASCO; National Comprehensive Cancer Network (NCCN) and NCCN are registered trademarks of NCCN. ASCO and NCCN do not endorse any product of therapy.
