How to interpret the Oncotype DX® test results?
For your patients with HR+, HER2-, early-stage, invasive breast cancer
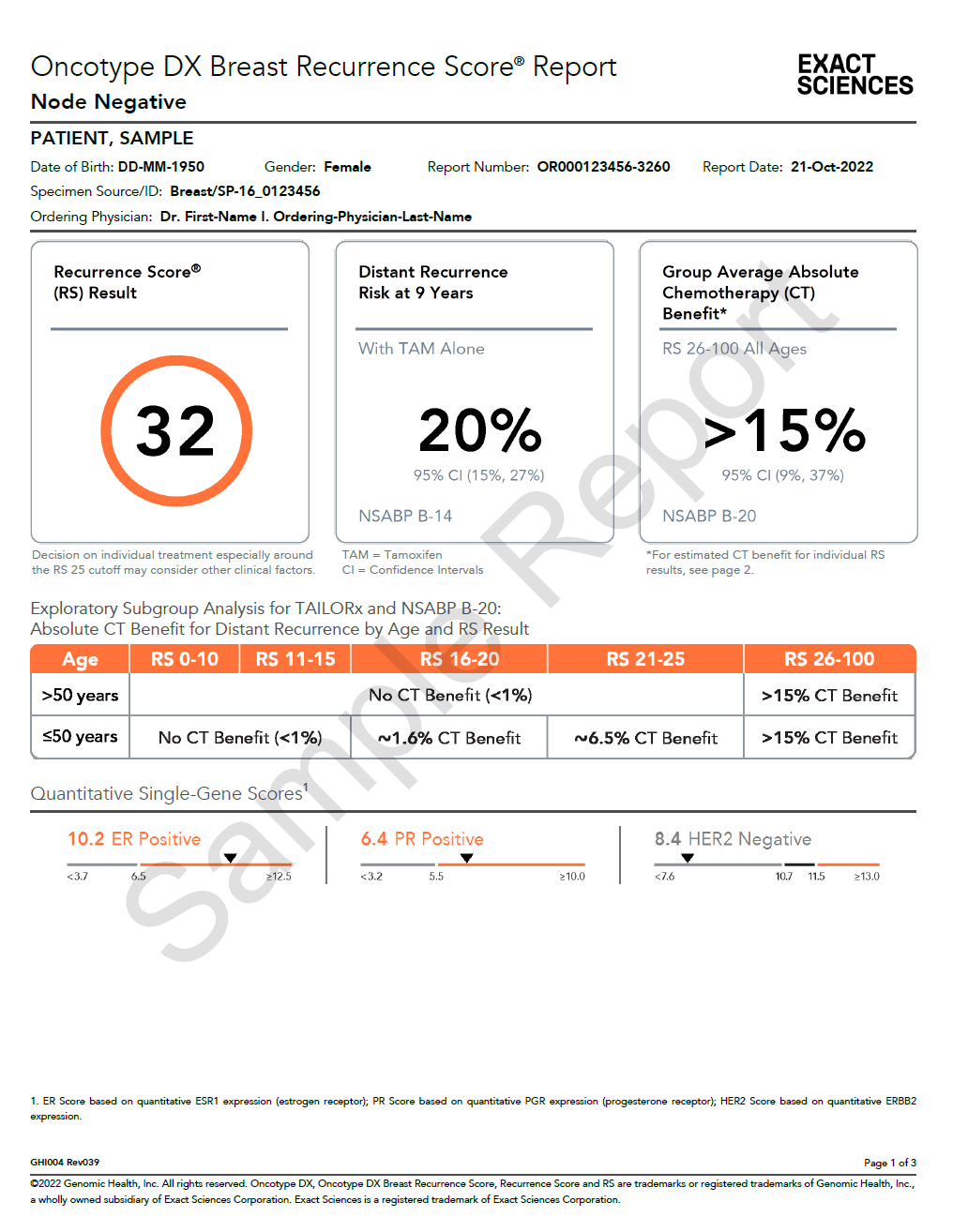
The Oncotype DX Breast Recurrence Score® report provides three points of clarity to aid in treatment decisions as to whether adjuvant chemotherapy is needed or not for node-negative and node-positive (N1: up to 3 positive lymph nodes), hormone receptor-positive, HER2-negative, early-stage breast cancer patients.
3 pieces of information provided by the test
1
Recurrence Score® result
This number, between 0 and 100, is calculated by measuring the activity of specific genes in the breast cancer tissue. The Recurrence Score result is used to predict the risk of the breast cancer returning at a distant site and whether chemotherapy may help reduce the risk.1-5
2
Distant recurrence risk at 9 yearsa
This percentage indicates the risk that the breast cancer will come back somewhere else in the body, “distant recurrence,” within 9 years (node-negative)1,6 or 5 years (node-positive)4,7,8 when treated with hormonal therapy alone for 5 years.
3
Absolute chemotherapy benefita
This percentage indicates the benefit expected for the Recurrence Score group from adding chemotherapy to hormonal therapy in order to reduce the risk of cancer recurrence or death.2-4, 6-8
-
Further Understanding the Results: Node-Negative Patients
-
Further Understanding the Results: Node-Positive Patients
-
ABBREVIATIONS
-
REFERENCES