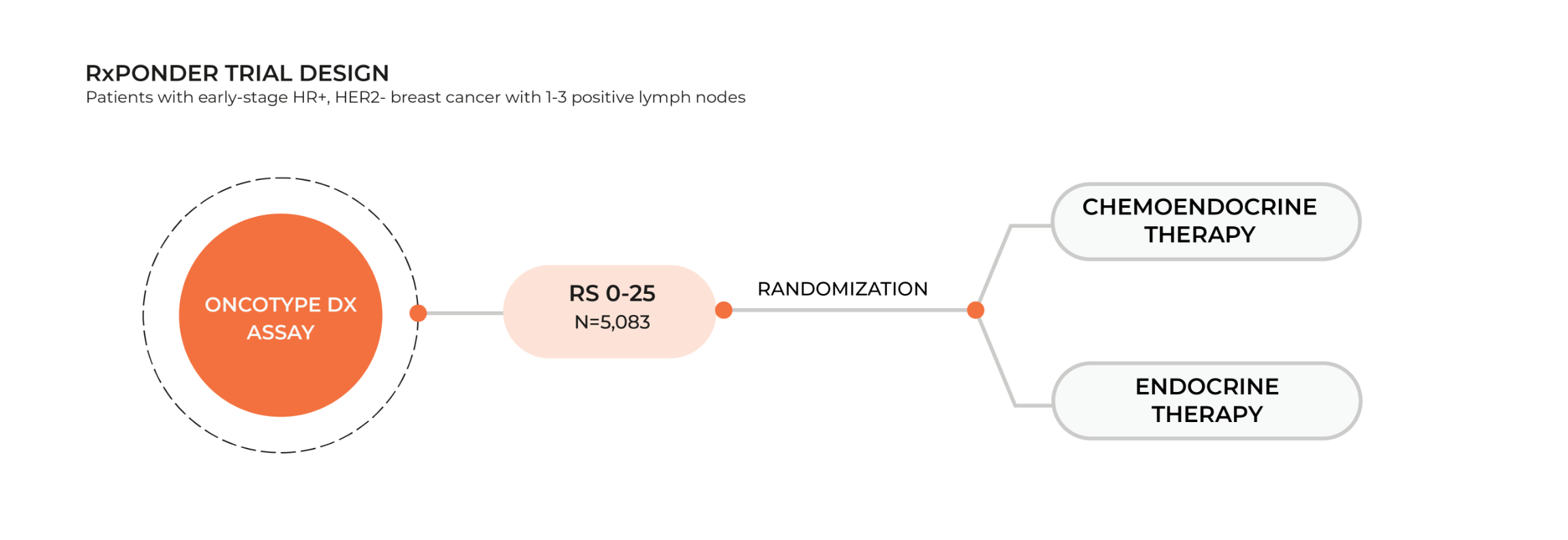
RxPONDER trial: expanding groundbreaking benefits to node-positive patients
The RxPONDER trial (A Clinical Trial RX for Positive Node, Endocrine Responsive Breast Cancer) prospectively randomized 5,083 HR+, HER2-, node-positive patients with Recurrence Score® (RS) results 0-25 to receive chemotherapy followed by endocrine therapy or endocrine therapy alone. The trial objectives were to8:
- Assess the chemotherapy benefit for these patients according to their Recurrence Score result
- Determine if the Oncotype DX® test predicts recurrence and guides treatment decisions
Most HR+, HER2-, N1, postmenopausal patients can be spared chemotherapy
Key findings from the study, led by the independent Southwest Oncology Group (SWOG) Cancer Research Network and sponsored by the National Cancer Institute (NCI), indicate that9:
- Patients with low Recurrence Score results can safely forgo chemotherapy without compromising survival rates
- Postmenopausal women with 1 to 3 positive nodes and Recurrence Score results 0-25 can forgo adjuvant chemotherapy regardless of clinical pathological parameters
- Premenopausal women with 1 to 3 positive nodes and Recurrence Score results 0-25 modestly benefit from chemotherapy
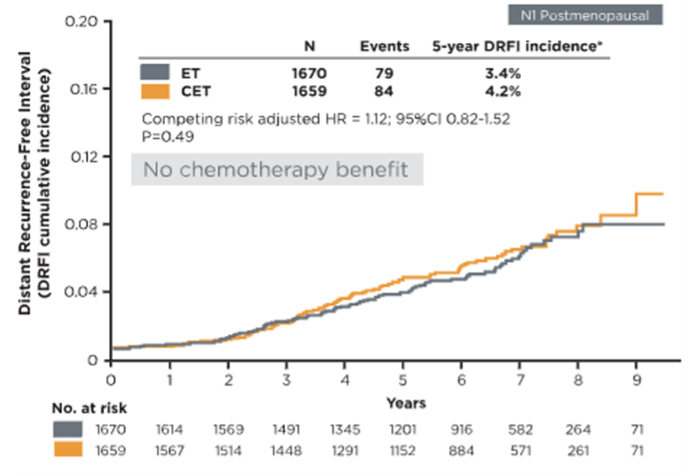
Safely spare postmenopausal patients from chemotherapy with confidence9
Postmenopausal women with Recurrence Score results 0-25 did not show benefit of chemotherapy in addition to endocrine therapy (competing risk-adjusted HR=1.12, 95% CI 0.82-1.52, P=0.49). Consistent lack of chemotherapy benefit was observed for invasive disease-free survival (IDFS) for subgroups of age, tumor size, grade, Recurrence Score result, and number of positive lymph nodes.
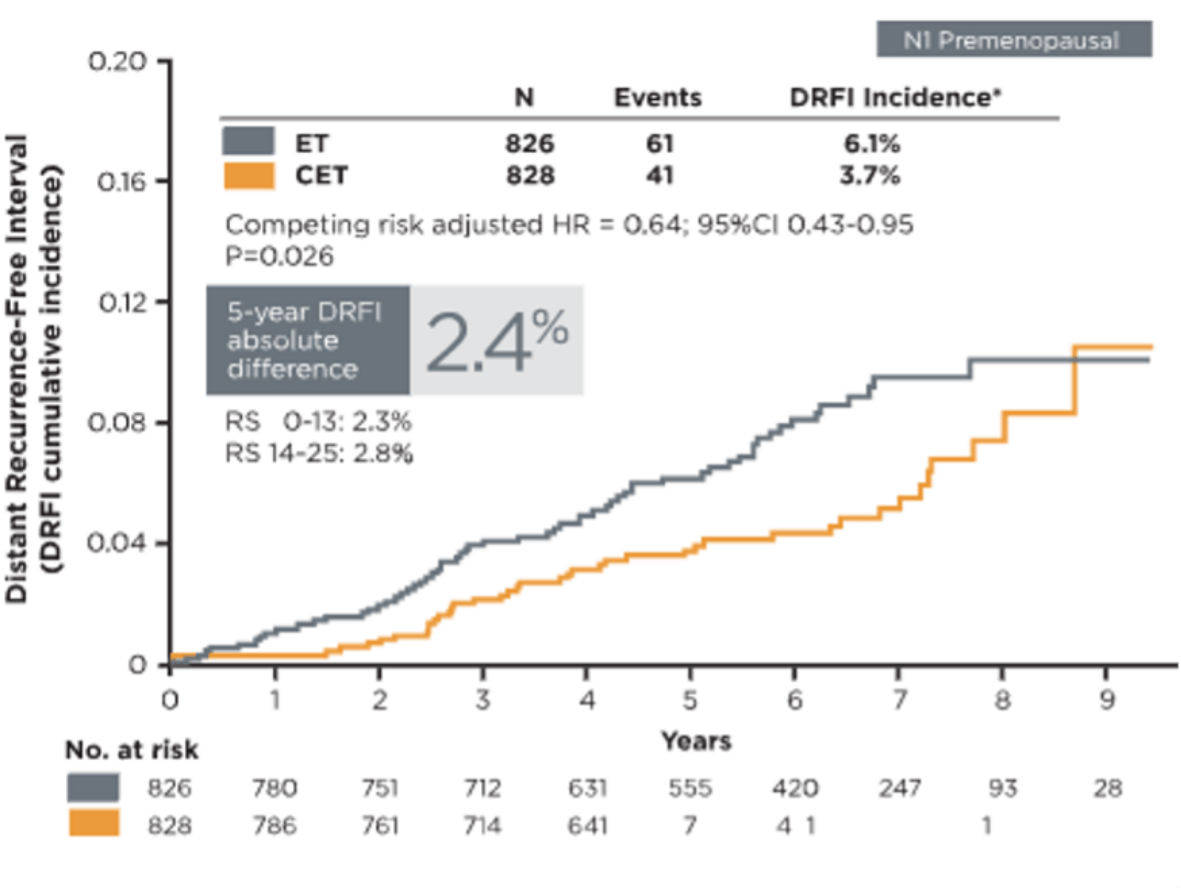
Determine chemotherapy options for N1 premenopausal patients with precision10
Premenopausal women with Recurrence Score results 0-25 had a significant benefit in disease recurrence-free interval (DRFI) from the addition of chemotherapy to endocrine therapy (competing risk-adjusted HR=0.64, 95% CI 0.43-0.95, P=0.026). Consistent benefit of chemotherapy was observed for IDFS for subgroups of age, tumor size, Recurrence Score result, and number of positive lymph nodes. The 5-year absolute benefit of chemotherapy for distant recurrence was 2.4% (RS 0-13: 2.3%; RS 14-25: 2.8%).
This 21-gene test is categorized by the NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) as “preferred” among gene expression assays for prognosis and prediction of chemotherapy benefit11
- Recommend to “strongly consider” for postmenopausal N1, HR+, HER2- patients with early-stage breast cancer who are candidates for chemotherapy (category 1 recommendation)
- Also to be considered for premenopausal N1, HR+, HER2- patients with early-stage breast cancer who are candidates for chemotherapy
The only test recognized by major guidelines for its high quality of evidence and robust strength of recommendation12-17
- Strongly recommended for N1 postmenopausal patients with ER+, HER2- early-stage breast cancer
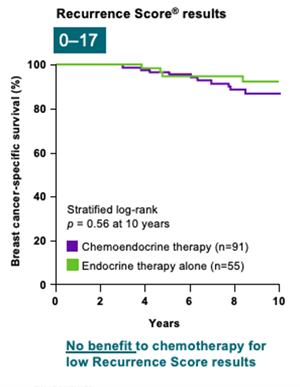
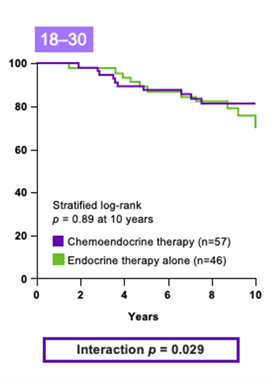
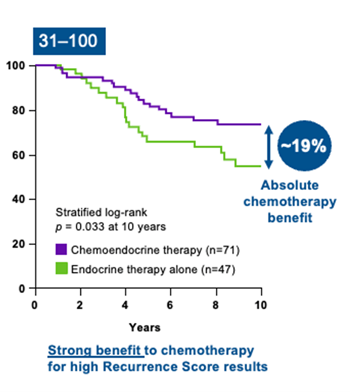
SWOG-8814 study
The SWOG-8814 study was performed on a cohort of 1,477 eligible postmenopausal patients with ER+, node-positive breast cancer.18 The likelihood of the Recurrence Score result to predict chemotherapy benefit was analyzed in 367 patients.3
Results from the study, led by the independent SWOG Cancer Research Network, indicate that:
- Recurrence Score result accurately predicts chemotherapy benefit in node-positive patients3
- There is no benefit to chemotherapy for those with low Recurrence Score results3
- There is a strong benefit to chemotherapy for high Recurrence Score results3
-
References
