
Oncotype DX Breast Recurrence Score
If you have recently been diagnosed with early-stage oestrogen receptor positive (ER+) invasive breast cancer, you will discuss your treatment options with your doctor*. As part of your treatment plan you might be given an option to have adjuvant chemotherapy (chemo) in addition to hormone therapy (tamoxifen or aromatase inhibitor). Choosing the optimal treatment for your breast cancer is important and you need to weigh the risks and benefits of chemotherapy with your doctor in order to make a decision that is right for you.
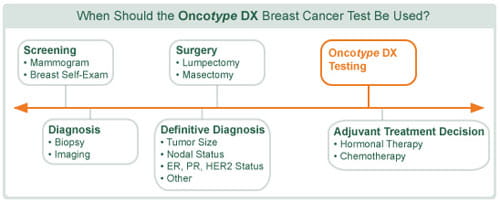
When considering the best adjuvant treatment option for early breast cancer, the doctor usually considers your age, general health and personal circumstances, as well as the size and grade of your tumour and whether any lymph nodes are involved. By examining the biology of the actual cells of your tumour, the Oncotype DX® test can add a valuable piece of information to these factors. The Oncotype DX test can thus help you and your doctor to make a more informed and personalised decision about whether to treat with chemotherapy or not, based on further information that is specific to the biology of your cancer.
The Oncotype DX test is a molecular diagnostic test that analyses the individual biology of a breast cancer tumour by examining the activity of 21 genes in the tumour tissue. The results of the analysis are fed into a formula that gives a Recurrence Score® result. The Recurrence Score result, a number between 0 and 100, can provide information about how likely your breast cancer is to recur within 10 years of diagnosis and, perhaps more importantly, the likelihood that you will benefit from chemotherapy.
As the Oncotype DX test is performed on a small amount of the tissue removed during your original surgery (core biopsy, lumpectomy, or mastectomy) this means you do not need to undergo any additional surgery or procedure in order to have the test.
Since becoming available in 2004, more than 900,000 Oncotype breast cancer tests have been requested in over 90 countries. The Oncotype test has been studied and validated in independent studies involving more than 4,000 invasive breast cancer patients.
The Oncotype DX test has been available in the UK and Ireland since the end of 2007, with usage in HSE, NHS and all private sectors growing. You can pay for the test yourself or it may be covered by your provider if you have private healthcare insurance. All of the major private insurance companies in the UK and Ireland now cover the test but you should always call your insurance company first just to check.
Note: Genomic Health is not able to advise you directly on your treatment plan.
*Breast surgeon or oncologist
During surgery the breast surgeon will remove the breast cancer and surrounding tissue and this is examined and preserved by the pathologist. The pathologist will send sections of the cancer tissue to the Genomic Health® laboratory (the company that created the Oncotype DX test). The Oncotype DX breast cancer test results are then reported to the requesting doctor* to help you and your doctor formulate a treatment plan based on the unique characteristics of your cancer.
There are no additional invasive procedures needed to obtain test results.
Your doctor is a valuable resource for helping you decide if the Oncotype DX test is right for you. Please note that Genomic Health is not able to advise you directly on your treatment plan.
*Breast surgeon or oncologist
The Oncotype DX breast cancer test was developed by a company called Genomic Health. Scientists at Genomic Health researched and developed a panel of 21 appropriate genes to test. 16 of these genes are associated with breast cancer; the other 5 were added to normalise expression for each patient. After analysing many hundreds of samples, the team developed a mathematical formula incorporating the expression level of each of these important genes. This formula results in one number, the Recurrence Score result, which predicts risk of recurrence for the patient. To verify its accuracy, this Recurrence Score has been validated in several clinical studies. To date, tumours from over four thousand patients in thirteen studies have been analysed. The gene expression analysis technology and the formula are two key components of the Oncotype DX breast cancer test.
Note: Genomic Health is not able to advise you directly on your treatment plan.

TAILORx Trial Reports Positive Results: 99% of women with low Oncotype DX Recurrence Score results are free of breast cancer recurrence after five years of hormone therapy alone.
On September 28, 2015, initial results were announced from the Trial Assigning IndividuaLized Options for Treatment (Rx), or TAILORx, a multi-center prospectively conducted trial of more than 10,000 women with early stage breast cancer sponsored by the National Cancer Institute (NCI), part of the National Institutes of Health, and led by the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) with support from Genomic Health, Inc. The study demonstrated that a group of trial participants with low 21-gene recurrence score (Oncotype DX Recurrence Score) results of 10 or less who received hormonal therapy alone without chemotherapy had less than a one percent chance of distant recurrence at five years.
This finding, published online by the New England Journal of Medicine, provides evidence that other women in the future may effectively use hormonal therapy alone if the Recurrence Score is 10 or less. Second primary cancers exceeded recurrences of the original breast cancer, resulting in 93.8 percent five year disease free survival, the primary trial endpoint.
“This prospective study involving uniformly treated patients with hormone-receptor-positive, HER2-negative, axillary node-negative breast cancer supports the clinical validity of the 21-gene assay in identifying patients who may be safely spared adjuvant chemotherapy.”1
For more information regarding the TAILORx trial, please visit the ECOG website.
Opened in January 2011, the RxPONDER Trial (Rx for Positive Node, Endocrine Responsive Breast Cancer) will reveal whether chemotherapy benefits patients with node-positive breast cancer who have low to intermediate Oncotype DX Recurrence Score result. The trial also seeks to determine whether there is an optimal Recurrence Score cutpoint for these patients, above which chemotherapy should be recommended.
The trial is being conducted by SWOG with the participation of all the major National Cancer Institute-funded cooperative groups in the United States.
Researchers plan to enroll 4,000 women with Recurrence Score results of 25 or less who have early stage, hormone receptor-positive, HER2-negative breast cancer that has been found to involve one to three lymph nodes. They expect to screen over 9,000 breast cancer patients to identify 4,000 with Recurrence Score results in this range.
The use of the Oncotype DX assay in patients with node-positive breast cancer has been validated in several studies including a previous SWOG led-study, SWOG 8814, ECOG 2197 and TransATAC. The test has been available for use by physicians in clinical practice for node-positive disease since 2008, and it has been reimbursed selectively in the U.S. for this patient population.
For further information regarding the RxPONDER trial, please visit the SWOG website.
REFERENCE:
1. Sparano et al. New Engl J Med. 2015. Prospective Validation of 21-Gene Expression Assay in Breast Cancer New Engl J Med
