Confidently plan a course of treatment based on clinically validated results.
The Colon Recurrence Score® test uses Exact Sciences’ well-established RT-PCR (reverse transcriptase-polymerase chain reaction) platform to offer individualized, quantifiable risk of recurrence for patients with colon cancer (anatomic stages II, MMR-P, or III A/B).4-7 With this information, you and your patient can more confidently plan a course of treatment based on clinically validated results.
Bolstering treatment recommendations in the SUNRISE-DI study8
Impact of the 12-gene recurrence score test on deciding adjuvant chemotherapy for stage II and IIIA/B colon cancer: the SUNRISE-DI study
- This study investigated the impact of the Oncotype DX Colon Recurrence Score test on adjuvant chemotherapy decision-making
- Physicians changed treatment recommendations in 45% in stage IIIA/B and 30% in stage II patients after receiving Colon Recurrence Score test results
- Physician confidence in their treatment recommendations significantly increased from 54% to 81% in stage IIIA/B and from 65% to 83% in stage II after receiving Colon Recurrence Score test results
Clinically validated in 4 studies with approximately 3000 patients
| Stage II Colon Cancer | Stage III Colon Cancer |
|
SUNRISE Trial Confirmed the prognostic capabilities of the test, beyond clinical and pathologic factors, in both stage II and stage III colon cancer7 View study details |
NSABP C-07 Trial Further confirmed the use of the test to stratify patients in stage II and validated the test as prognostic of risk of recurrence in stage III colon cancer6 View study details |
|
CALGB 9581 Confirmed the ability to stratify patients by risk5 View study details |
QUASAR Study Found that the test is strongly associated with the risk of recurrence and provides information beyond clinical and pathological factors4 View study details |
SUNRISE trial findings
The SUNRISE retrospective cohort study included archived tissue from 597 stage II and stage III Japanese patients treated with surgery alone. The SUNRISE study7:
- Confirmed the prognostic capabilities of the Colon Recurrence Score test in stage II colon cancer
- Was the first clinical validation of the test as a prognostic marker of recurrence risk in patients with stage III colon cancer treated without adjuvant chemotherapy
- Provided new insight into the natural history of stage III colon cancer treated without chemotherapy and showed the heterogeneity in risk of recurrence
Recurrence Risk Estimated by Oncotype DX Risk Group and Disease Stage
Stage II Disease
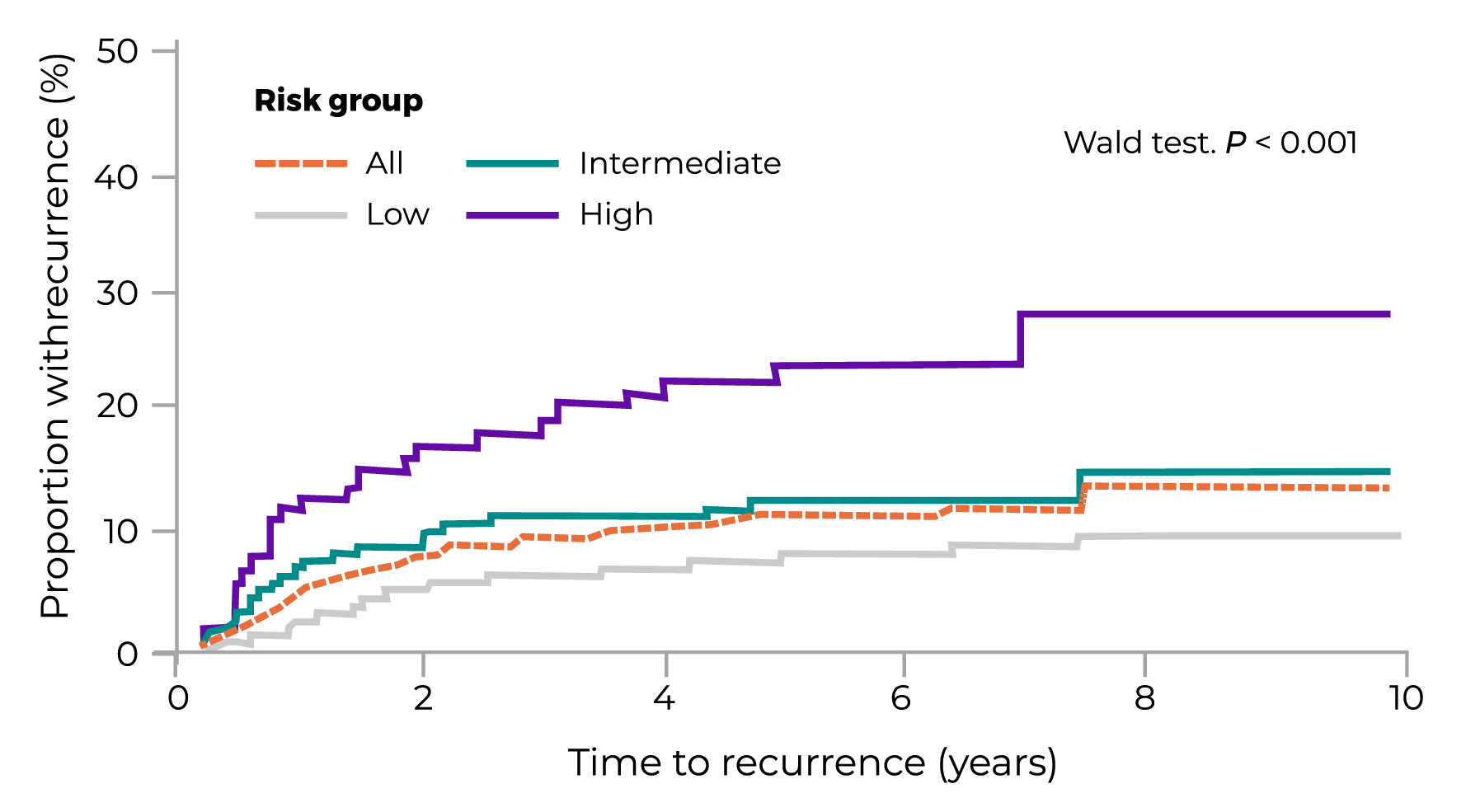
| RISK GROUP | % OF PATIENTS | 5-YEAR RECURRENCE % (95% CI) |
| All | 100 | 11 (9 to 14) |
| Low | 60 | 8 (6 to 11) |
| Intermediate | 26 | 13 (8 to 19) |
| High | 14 | 24 (17 to 34) |
Stage III A/B Disease
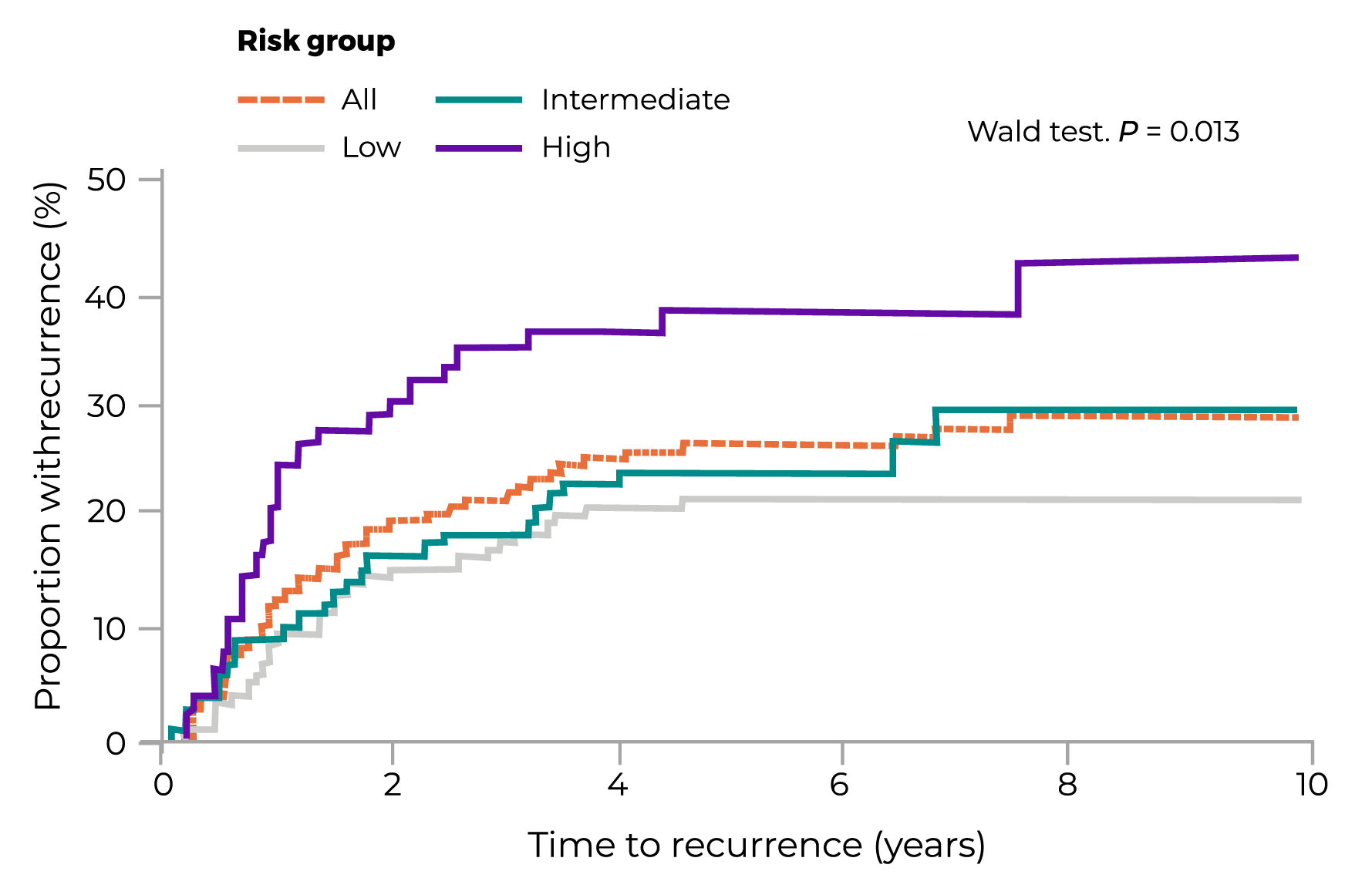
| RISK GROUP | % OF PATIENTS | 5-YEAR RECURRENCE % (95% CI) |
| All | 100 | 27 (22 to 32) |
| Low | 46 | 22 (16 to 30) |
| Intermediate | 31 | 25 (17 to 35) |
| High | 23 | 39 (28 to 51) |
CALGB 9581 study findings
Consistent with QUASAR, in the CALGB 9581 study, the Recurrence Score® result was shown to be prognostic for recurrence risk, supporting the results of the first validation study. This study featured 690 stage II colon cancer patients perceived to be low risk (based on the clinical and pathologic characteristics), with an average 5-year risk of recurrence of 13%.5
- Is a significant predictor of risk of recurrence (P=0.004) in multivariable analysis, providing independent value beyond conventional clinical and pathologic factors
- Identified 22% of the patients in the high Recurrence Score test group, with an average 5-year recurrence risk of 21%
- Therefore, the test can discriminate a higher-risk patient population beyond conventional measures—even in a cohort of perceived low-risk patients
NSABP C-07 study findings
This independent, prospectively designed, validation study of the Colon Recurrence Score test included 892 stage II and stage III patients randomized to 5-FU/LV or 5-FU/LV plus oxaliplatin. The NSABP C-07 trial6:
- Validated the test as prognostic of recurrence risk in patients with stage III colon cancer
- Confirmed the ability of the Recurrence Score result to quantify the risk of recurrence in stage II and III patients treated with either 5-FU/LV or 5-FU/LV + oxaliplatin
- Predicted risk of recurrence beyond conventional measures, including T and N stage, and MMR status
QUASAR Study Findings
The first validation study, QUASAR, was prospectively designed and included archived tissue from 1,436 stage II colon cancer patients randomized to surgery or surgery + 5-FU/LV. The landmark QUASAR study showed4,9:
- A significant association between the continuous Recurrence Score result and the risk of recurrence (P=0.004)
- In the multivariate analysis of patients randomized to surgery alone, the most significant independent predictors of risk of recurrence were:
- Recurrence Score result
- Mismatch Repair (MMR) status
- T-stage
- T3, MMR-P patients with Recurrence Score result ≥41 have similar risk to T4, MMR-P patients and may have a higher absolute benefit from adjuvant chemotherapy
In Stage II patients following surgery, a Recurrence Score result predicts individual recurrence risk4
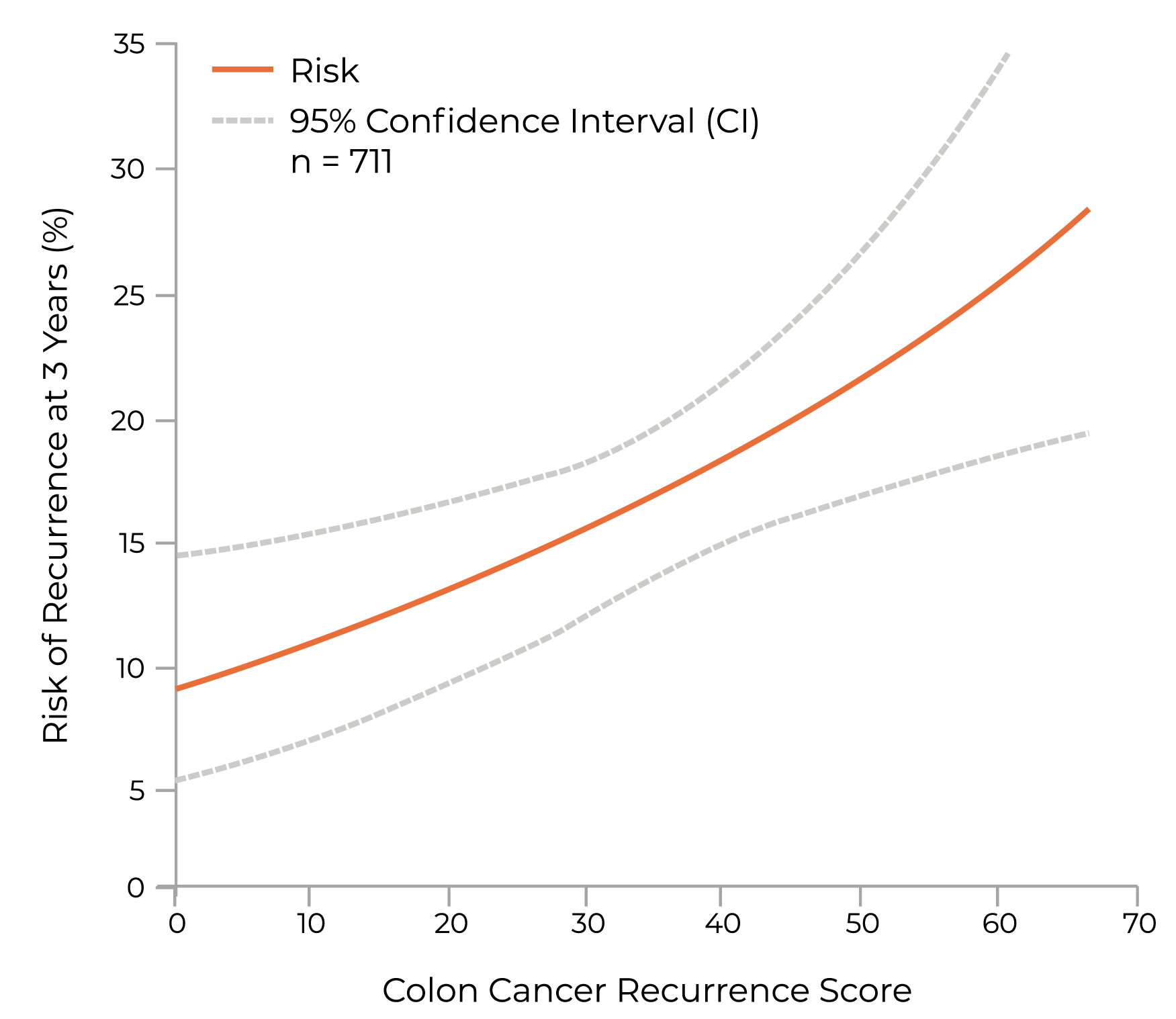
Validated as an independent, quantitative assessment of recurrence risk in Stage II colon cancer—QUASAR Study9
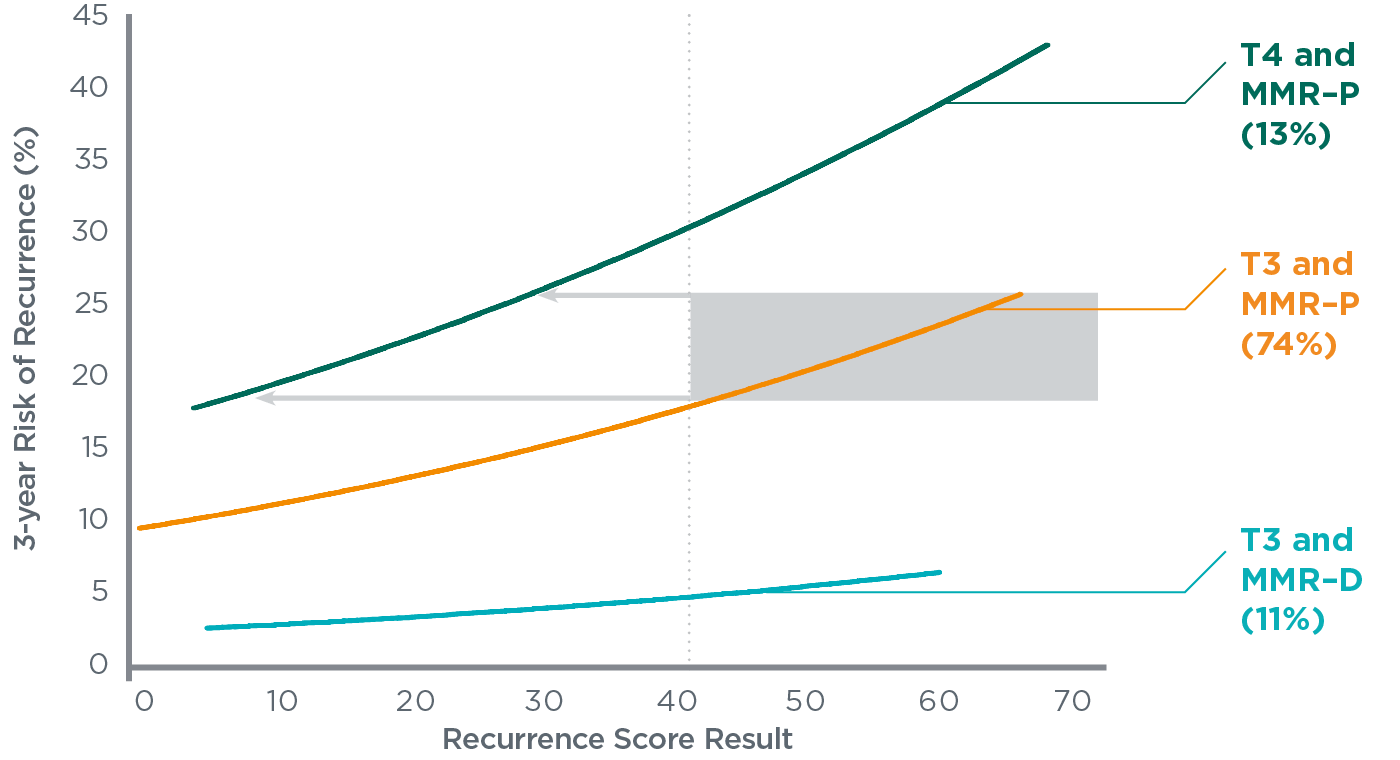
- Patients with a Recurrence Score result ≥41 had recurrence risk >18%, similar to some T4, MMR-P patients
- Therefore, these patients are expected to have higher absolute benefit from 5-FU/LV compared to those with low Recurrence Score results
-
References
